AP®︎ Chemistry: Group trend for ionization energy
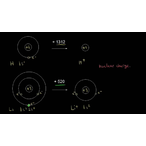
Explaining trend in ionization energy down a group using concepts of nuclear charge, electron shielding, and distance of electrons from nucleus
AP®︎ Chemistry
An introductory college-level chemistry course that explores topics such as atoms, compounds, and ions; chemical reactions and stoichiometry; ideal gases; chemical equilibrium; acids and bases; kinetics; thermodynamics; redox reactions and electrochemistry; and a whole lot more!
Periodic table trends
Have you ever wondered why it is called the "periodic" table? The elements are organized based on their electronic structure, which results in some interesting trends in atomic properties as you move down a group or across a period. This t…
There are no frequently asked questions yet. If you have any more questions or need help, contact our customer service.
Explaining trend in ionization energy down a group using concepts of nuclear charge, electron shielding, and distance of electrons from nucleus
AP®︎ Chemistry
An introductory college-level chemistry course that explores topics such as atoms, compounds, and ions; chemical reactions and stoichiometry; ideal gases; chemical equilibrium; acids and bases; kinetics; thermodynamics; redox reactions and electrochemistry; and a whole lot more!
Periodic table trends
Have you ever wondered why it is called the "periodic" table? The elements are organized based on their electronic structure, which results in some interesting trends in atomic properties as you move down a group or across a period. This tutorial will try to explain some of those trends!
Topic: Science
Learn about all the sciences, from physics, chemistry and biology, to cosmology and astronomy, across hundreds of videos, articles and practice questions.
There are no frequently asked questions yet. If you have any more questions or need help, contact our customer service.